New frontiers for milk proteins: functional foods and drug delivery systems
The ability to control and manipulate protein behaviour at an interface can influence a broad range of technological processes and physicochemical and biological phenomena. In some cases, such as the development of pharmaceutical products and functional emulsion-based food, protein adsorption is a desirable feature that enhances the end product. In other situations adsorption is undesirable, for example the adherence of proteins to artificial prostheses implanted in the human body resulting in adverse reactions, or contact lens fouling leading to reduced performance.
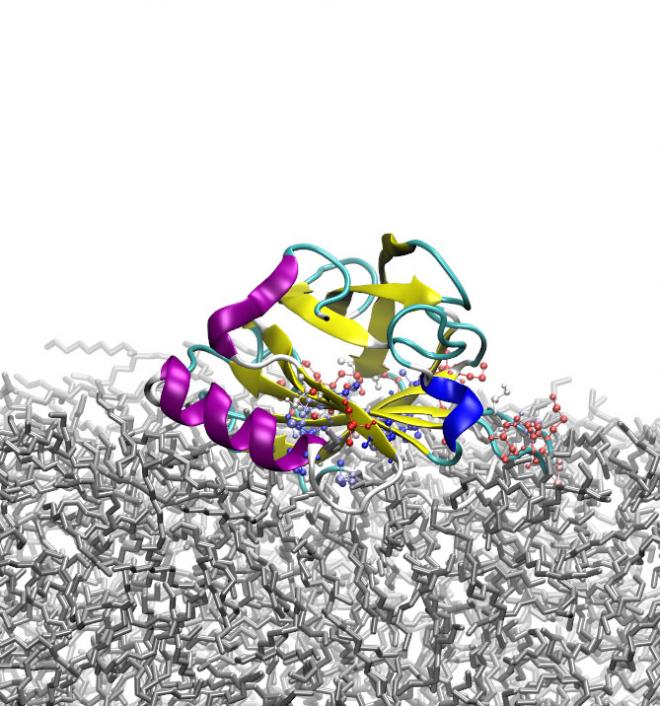
Although many experimental studies have contributed to rapid progress in the fundamental knowledge of protein behaviour at interfaces, detailed molecular-level understanding of the mechanism of protein adsorption at an interface is still remarkably lacking. The high performance computing resources available through NeSI allowed Davoud Zare of Victoria University of Wellington, supervised by Prof. Kate McGrath (MacDiarmid Institute/Riddet Institute/Victoria University) and Dr Jane Allison (Massey University), to use atomistic molecular dynamics simulations to characterise the adsorption of the milk protein β-lactoglobulin (β-LG) at three different oil/water (O/W) interfaces. These involved either the hydrophobic oil decane, the marginally hydrophilic oil octanol, or the more hydrophilic oil triolein.
Each simulation comprised a periodic cubic box containing one molecule of β-LG (1604 atoms), nine sodium ions and large oil and water phase regions, giving 64,115 – 124,367 atoms, or interacting particles, in total for each system. As the pairwise interactions between the particles must be evaluated at each integration step, the computational cost scales up enormously as system size increases. In addition to these being large simulation systems, it was also necessary to test whether the initial orientation of β-LG with respect to the oil surface affected its adsorption, and to run each simulation for sufficiently long for stable adsorption to occur. At least two independent simulations of each of three different initial orientations were run for each oil, making 22 simulations in total. Finally, separate simulations of each type of oil interfaced to vacuum and to water were also run to verify that the simulations accurately capture the interfacial tensions of the various phase combinations. Each simulation was run for 200 ns, with an integration time-step of 2 fs, requiring 100,000,000 integration steps. The NeSI Postgraduate allocation class allowed these simulations to be carried out using the GROMACS MD software across 192 cores on the University of Auckland Pan cluster or 64 cores on the University of Canterbury Power755 cluster.
The simulations showed that both the approach to the surface and the mechanism of adsorption of β-LG to the O/W interface depend upon the hydrophilicity of the oil and the interfacial tension of the O/W interface, with the nature of the adsorption, the accompanying structural changes and the energetic driving force differing markedly between the different oils. Intriguingly, the behaviour of the protein was also found to resemble that predicted for a soft spherical particle at an O/W interface.
In the decane/water system, the protein flattens itself against the decane surface, exposing its hydrophobic interior residues to the hydrophobic surface. This is in keeping with the experimental observation that proteins undergo more conformational change upon adsorption to hydrophobic surfaces, and tend to flatten out to create a thin protein layer. In contrast, β-LG buries itself entirely in the marginally hydrophilic octanol, without markedly changing its secondary or tertiary structure. Finally, in the presence of triolein, the most hydrophilic oil tested, the protein partially inserts itself into the oil phase. By controlling the nature of the oil, in particular its hydrophilicity and the interfacial tension of the oil/water interface, it is therefore possible to control the way in which β-LG adsorbs to the interface.
Not only did the nature of the adsorption observed in the simulations largely agree with experimental findings, the simulations revealed the structural and mechanistic detail behind each mechanism of adsorption, paving the way for the use and engineering of protein-stabilised emulsions as functional food products and drug delivery systems.